Description
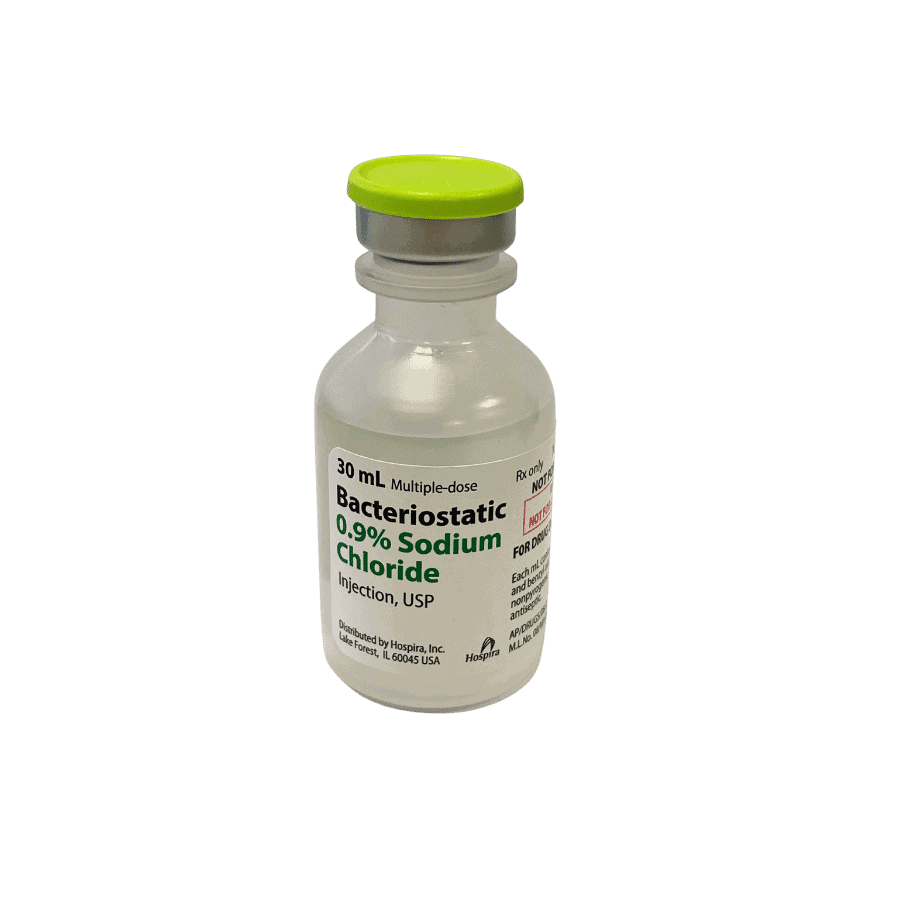
Bacteriostatic 0.9% Sodium Chloride 30ml
Hospira Bacteriostatic Sodium Chloride for injection USP is a sterile, multiple dose vial that repeated withdrawals may be made from.
Each milliliter (ml) contains sodium chloride 9 mg and 0.9% (9 mg/ml) benzyl alcohol added as a bacteriostatic preservative.
May contain hydrochloric acid for pH adjustment. The pH is 5.0 (4.5 to 7.0).
More Information
Please note this is an UNLICENSED product and as such the prescriber takes full responsibility for prescribing the medicine and for overseeing the patients care, monitoring, and any follow up treatment.
An UNLICENSED product should be used where a suitable LICENSED product does not exist.








Heather Reynolds (verified owner) –
Teleta pharmacy outstanding products outstanding service impeccable delivery times….never a problem..Heather The Beauty Spot Prestatyn xx
sfaesthetics20 (verified owner) –
Majority time have ample stock if everything, always manage next day delivery and never have any problems ????
C Jones (verified owner) –
Great value, quick delivery. Happy customer
topbrazilianuk –
How can I buy it if doesn’t have a bottom to check out it?
Teleta Support –
Hi, Bacstat is out of stock I’m afraid. I’m checking why the notice has not displayed